PRODUCTS
Minimal Residual Disease (MRD)
MRD is the presence of low levels of malignant cells that remain in a subject during or after treatment or when the patient is in remission. Detectable levels of cancer cells following treatment may suggest a higher probability of relapse.
PRODUCTS
Minimal Residual
Disease
(MRD)
MRD is the presence of low levels of malignant cells that remain in a subject during or after treatment or when the patient is in remission. Detectable levels of cancer cells following treatment may suggest a higher probability of relapse.
MRD Solutions
LabPMM services include MRD tracking of targeted mutations including FLT3 ITD and NPM1, or LymphoTrack® enabled Ig/TR rearrangement detection. Invivoscribe product offerings include the LymphoTrack® portfolio of solutions for MRD tracking of B-cell/T-cell clonality.
MRD Solutions
LabPMM services include MRD tracking of targeted mutations including FLT3 ITD and NPM1, or LymphoTrack® enabled Ig/TR rearrangement detection. Invivoscribe product offerings include the LymphoTrack® portfolio of solutions for MRD tracking of B-cell/T-cell clonality.
LymphoTrack® MRD NGS
NGS Powered Assays, Controls & Software for Longitudinal MRD Testing
The RUO T-cell MRD Bundle includes:

The RUO B-cell MRD Bundle includes:
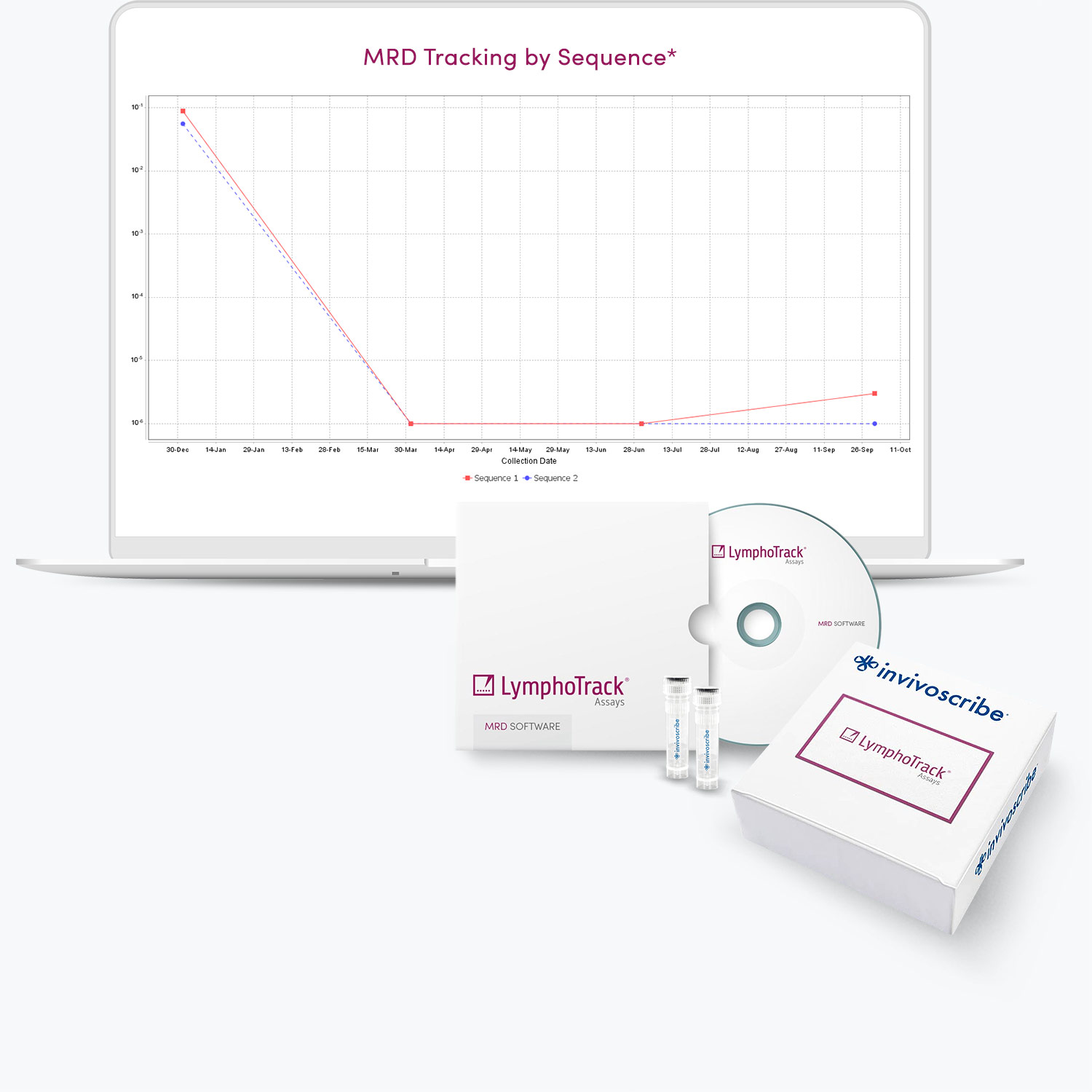
* LymphoTrack MRD Bioinformatics Software
- Proprietary standalone software enables trending over time.
- Includes a module to design experiments that can detect as little as one cancer cell in one million healthy cells (with sufficient DNA input).
- Claim MRD negativity with the desired level of confidence.
- Representative graph will be included in the upcoming software version.
Create a tailored NGS Solution to assess MRD. Consult with our hematology oncology specialists.
